Answer:
True. it is restricted to the orbits of certain radii around the nucleus.
The position can change, but it must be in one of these orbits
Step-by-step explanation:
In this exercise some affirmations are given and you must select which ones are correct, for this we review the Bohr atomic model that has the following postulates:
* the orbits are circular
* Only certain orbits are stable, stationary state
* the radiation emitted is the difference in energy between two stable orbits
* the size of the orbit is given by the quantization of the angular momentum
L = n
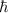
When reviewing the different statements, the correct one is:
* it is restricted to the orbits of certain radii around the nucleus.
The position can change, but it must be in one of these orbits