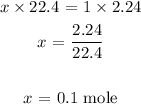Answer:
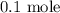
Step-by-step explanation:
Here, we want to get the number of moles in 2.24 L of a sample of gas
Mathematically,
At STP, 1 mole of a gas occupies a volume of 22.4 L
Thus x moles will occupy 2.24 L at STP
To get the value of x, we have it that: