ANSWER
The arrow will be shifted to the right (OPTION B)
Step-by-step explanation:
Firstly, we need to write out the chemical reaction equation
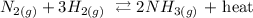
From the reaction above, you will see that heat is one of the products, this means that the reaction is n exothermic reaction.
An exothermic reaction is a type of reaction in which heat is released to its surroundings.
In an exothermic reaction, a decrease in temperature will shift the equilibrium to the right