The pH value indicates the concentration of H+ ions in the solution. We are given the value of the concentrations of the OH- ions, so we can find the pOH using the following equation:
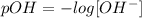
The sum of pH and pOH will always be equal to 14, so we find the pH by clearing it from the following relationship:
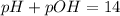
So, pOH value will be:
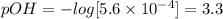
The pH will be:
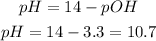
The pH of the solution will be 10.7