Ratios and percents
The total number of squares is 6x6 = 36
the number of squares of the shaded area is 3x4 = 12
Ratio
We find the ratio by dividing those two quantities:
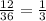
Percentage
We find the percentage by multiplying the result by 100%
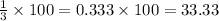
Answer: C. 1/3 = 33.33%