Answer:
332.19 years
Explanation:
The weight, W of the substance after n years is given by:
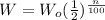
Let the initial weight = 100%
If the substance loses to 10% of its initial weight, then:
• Wo = 100%
,
• W= 10%
Substitute these into the formula:
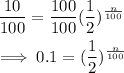
We then solve the equation for the value of n.
Take the logarithm of both sides.
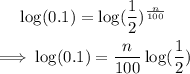
Then divide both sides by log(1/2):
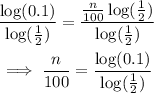
Finally, multiply both sides by 100:
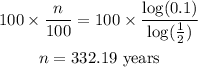
It will take at least 332.19 years for the radioactive substance to lose to 10% of its initial weight.