Answer:
2, 3, 1, 3.
Step-by-step explanation:
Remember that a balanced chemical equation is when we have the same number of elements for reactant and product side.
Let's see the unbalanced equation:
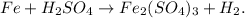
You can note that we have:
You can realize that we have Fe, S, and O unbalanced, but if we put '2' moles beside Fe, we will balance Fe, like this:
![2Fe+H_2SO_4\operatorname{\rightarrow}Fe_2(SO_4)_3+H_2]()
Now, if we put '3' moles beside H2SO4 we will balance S and O, obtaining 3 moles of S, and 12 moles of O for both sides:
![2Fe+3H_2SO_4\operatorname{\rightarrow}Fe_2(SO_4)_3+H_2]()
But H is unbalanced because on the left side we have 6 hydrogens but on the right side we have 2 hydrogens, so if we put '3' moles beside H2, we obtain the balanced chemical equation:
![2Fe+3H_2SO_4\operatorname{\rightarrow}Fe_2(SO_4)_3+3H_2.]()
The order of the coefficients is 2, 3, 1, 3.