Given,
Addition of heat, Q=+754.3 J
Work done , W=-424.5 J
The work is done against constant pressure.
To find
The value of E for this process
Step-by-step explanation
According to the first law of thermodynamics,
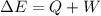
Putting the values,
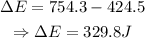
Conclusion
The required value is 329.8 J