Answer
5.299 grams
Step-by-step explanation
Given:
Concentration of Cl⁻ = 0.059 M
Volume of solution = 462 mL = 0.462 L
What to find:
The grams of NiCl₂ in 462 mL solution
Step-by-step solution:
The first step to write a balanced equation for the dissolution of NiCl₂:
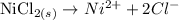
From the balanced equation; 1 mole of NiCl₂ produces 2 moles of Cl⁻
Given that the concentration of Cl⁻ = 0.059 M, so the concentration of Ni⁺ will be:
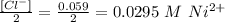
So that Molarity, M, of NiCl₂ = 0.059 M + 0.0295 = 0.0885 M
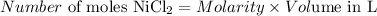
Number of moles of NiCl₂ = 0.0885 M x 0.462 L = 0.040887 mol
The last step is to convert the number of moles of NiCl₂ to grams using the formula below:
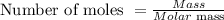
From the Periodic Table;
Molar mass of NiCl₂ = 129.5994 g/mol
So, mass of NiCl₂ in 462 mL solution = 0.040887 mol x 129.5994 g/mol = 5.299 grams