Answer:
Na2S2O3.
Step-by-step explanation:
What is given?
If we suppose that the compound has a mass of 100 g:
Mass of Na = 29.1 g,
Mass of S = 40.5 g,
Mass of O = 30.4 g,
Molar mass of Na = 23 g/mol,
Molar mass of S = 32 g/mol,
Molar mass of O = 16 g/mol.
Step-by-step solution:
First, we have to convert all the given masses of each element to moles using their respective molar mass, like this:
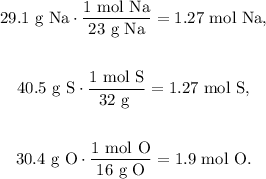
The next step is to divide each number of moles by the least number of moles obtained, which in this case is 1.27, as follows:
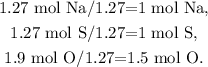
Remember that we need to have the number of moles of each element as integers, so if we multiply each number of moles of each element by 2, we will obtain:
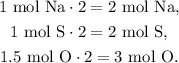
We would have 2 mol of Na, 2 mol of S, and 3 mol of O, so the empirical formula is Na2S2O3