ANSWER
Endothermic reaction
Step-by-step explanation
Given that;
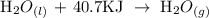
In the reaction above, there is a transition from liquid water to steam. This phase change is called boiling.
During the process, heat is absorbed from the surroundings.
Therefore, the reaction is an endothermic reaction.