Answer;
0.168M
Explanations:
The formula for calculating the concentration of the solution is expressed as:
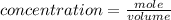
Determine the moles of iron(II) chloride
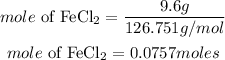
Given that volume is 0.45L, hence the concentration is expressed as:
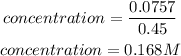
Hence the concentration of the solution is 0.168M