To find the amount represented by 1.5x10^16 molecules of BF3 we have to use Avogadro's Number. It was stated that 1 mol of any substance contains 6.022x10^3 atoms or molecules of that substance, that is what we know as Avogadro's Number:
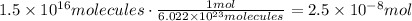
The answer is 2.5x10^-8 moles of BF3.