We can always convert mass (g) to moles by using the molar mass of the substance, which can be found in the periodic table. In this case, we will use the molar mass of sodium (Na), which is 23g/mole, i.e., each 23g of sodium corresponds to 1 mole.
Since we want to find the mass of 1.8 moles of Na, we can set the following proportion:
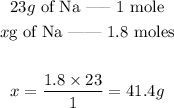
So, by using 1.8 moles of Na, we are using 41.4 g of sodium (Na).