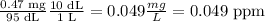Answer & Procedure
To solve this problem you will need to transform all the concentrations to the equivalence of ppm, which is 1 mg of solute per 1 L of solution.
Calcium: There are 43 μg of calcium in a total volume of 87 mL.
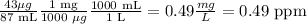
Caffeine: There is 0.91 mg of caffeine in a total volume of 105 mL.
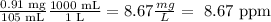
Trace particles: There is 0.47 mg of trace particles in a total volume of 95 dL.