ANSWER:
Zero
Explanation:
Heat (Q) and work (W) are the two ways to add or remove energy from a system. The processes are very different, however, both can change the internal energy (ΔU) of a system.
It is given by the following equation:
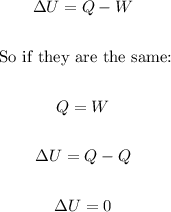
The net change in internal energy (ΔU) is zero