Answer
The resulting solution will be neutral.
Step-by-step explanation
Given:
Volume of HClO4 = 60.0 mL = 0.06 L
Molarity of HClO4 = 0.0500 M
Volume of NaOH = 40.0 mL = 0.04 L
Molarity of NaOH = 0.0750 M
What to find:
To determine if the resulting solution from (HClO4 and NaOH) acidic, basic, or neutral.
Solution;
The first step is to write a balanced chemical equation for the reaction.
HClO4 + NaOH → NaClO4 + H2O
The reaction is a neutralization reaction (also a double displacement reaction). The strong acid (HClO4) and a strong base react to produce salt (NaClO4) and water (H2O).
The next step is to determine the moles of the acid and the base using the formula below:
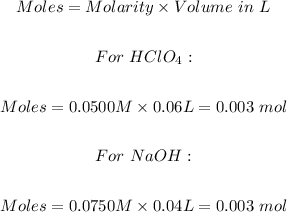
From the balanced chemical equation, 1 mol HClO4 neutralized 1 mol NaOH.
Therefore, 0.003 mol HClO4 will completely neutralize 0.003 mol NaOH and the resulting solution will be neutral.