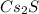INFORMATION:
We have the following ionic compounds:
- barium sulfate
- lithium nitrate
- cesium sulfide
And we must write their formulas
STEP BY STEP EXPLANATION:
1. Barium sulfate:
To write its formula, we need to use that:
- the element symbol for Barium is Ba, and it has a charge of 2+
- in the given table, sulfate is a polyatomic ion represented by SO4, and it has a charge of 2-
Now, we must add up the charges to verify if the ionic compound is neutral
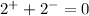
Since the compound has a net charge of zero, we can write its formula as
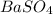
2. Lithium nitrate:
To write its formula, we need to use that:
- the element symbol for Lithium is Li, and it has a charge of 1+
- in the given table, nitrate is a polyatomic ion represented by NO3, and it has a charge of 1-
Now, we must add up the charges to verify if the ionic compound is neutral
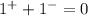
Since the compound has a net charge of zero, we can write its formula as
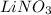
3. Cesium sulfide:
To write its formula, we need to use that:
- the element symbol for Cesium is Cs, and it has a charge of 1+
- the element symbol for sulfide is S, and it has a charge of 2-
Now, we must add up the charges to verify if the ionic compound is neutral
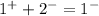
Since the compound has a net different from zero, we need to analyze the compound.
So, if we have just one atom of Cs, the charge would be 1+, but if we take two atoms the charge would be 2(1+) = 2+
Now, verifying the net charge
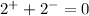
Now, we can write its formula as
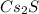
ANSWER:
barium sulfate:
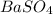
lithium nitrate :
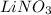
cesium sulfide: