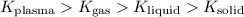According to the Kinetic Molecular Theory, the states of matter of a substance depend on the temperature, which is greater as the kinetic energy of the particles that form that substance is also greater.
A solid is the result of low kinetic energy of the molecules of the susbtance, as they remain mostly at the same place.
A liquid is the result of middle kinetic energy of the molecules of the substance, as they move relative to each other.
A gas is the result of high kinetic energy of the molecules, as they can move freely through all the space.
A plasma is the result of the ionization of molecules of a gas, which results in the production of light due to the high temperature that it reaches.
Then, in order of highest kinetic energy to lowest kinetic energy:
Gas: 2
Solid: 4
Plasma: 1
Liquid: 3