Answer:
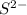
Step-by-step explanation:
Here, we want to know the possible ion with which aluminium forms the hypothetical compound
As we know, aluminium is a metallic compound and thus have a positive ion. The charge on this ion is +3
This is evident from the hypothetical compound
However, we can see that for a compund to be formed, we need an interchanging of ions and that would serve as the subscript
From the hypothetical compound, we can see that the subscript is 2
That automatically means that we need an ion with a -2 charge
Looking at the option, we can see this in S2-
This will serve as the correct choice for X