We are asked to determine DeBroglie's wavelength of a bullet. Let's remember that DeBroglie's wavelength is the ratio of Plank's constant and the momentum of the particle, therefore, we have the following formula:
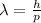
Where:

The momentum of the particle is the product of the mass and the velocity, therefore, we have:
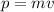
Substituting in the formula for the wavelength:
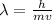
Now, we substitute the values:
![\lambda=\frac{6.63*10^(-34)J\cdot s}{(0.075\operatorname{kg})(350(m)/(s))}]()
Now we solve the operations:

Therefore, DeBroglie's wavelength is 2.5 x 10 ^-35 meters.