Answer:
Equilibrium will proceed in the left direction.
Explanations
Given the balanced chemical reaction;
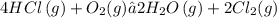
Increased volume gases results in decreased pressure. Therefore if the volume of the gas is increased, the equilibrium position will shift to the side with more moles of gas.
Since there are more moles of gases at the reactant than product, hence the reaction equilibrium will proceed in the left direction.