ANSWER
The actual yield is 125.646 grams
Explanation
Given information
The mass of sodium in grams = 57.50 grams
The percentage yield = 86%
The next step is to write the balanced equation of the reaction
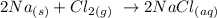
From the above reaction, we can see that 2 moles of sodium react with 1 mole of chloride too give moles of sodium chloride
The next step is to find the number of moles sodium using the below formula
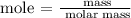
According to the periodic table, the molar mass of sodium is 23 g/mol
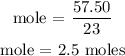
The next step is to find the number of moles of sodium chloride using a stoichiometry ratio
From the above reaction, you can see that 2 moles of sodium react to give 2 moles of sodium chloride
Let x represents the number of moles of sodium chloride
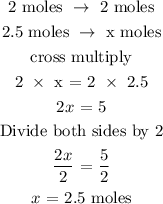
Therefore, the number of moles of NaCl is 2.5 moles
The next step is to find the theoretical yield using the below formula
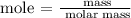
According to the periodic table, the molar mass of NaCl is 58.44 g/mol
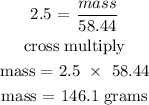
Since the theoretical yield is 146.1 grams, then we can now find the actual yield using the below formula
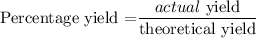
Recall that, the percentage yield is86%

Therefore, the actual yield is 125.646 grams