Answer:
27.86 days
Explanation:
The amount, A(t) of a radioactive substance left after time t is modeled by the equation below:
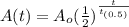
Given:
• Initial Substance, Ao = 18 grams
,
• Half-Life, t(0.5) = 12 days
,
• Amount, A(t)=20% of 18 grams
Substitute into the formula above:
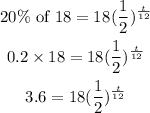
We solve the equation for t below.
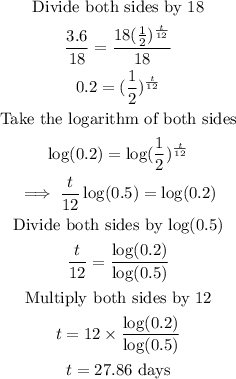
After 27,86 days, there will be 20% of the initial substance remaining.