The chemical equation is
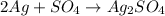
According to the equation, the ratio between Ag and the product is 2:1.
So, if we have 0.50 moles of Ag, then we use the ratio to find the moles of the product.
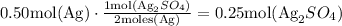
Therefore, there can be formed 0.25 moles of Ag2SO4 from 0.50 mol of Ag.