Answer:
The enthalpy change for equation A is -1287.5kJ.
Step-by-step explanation:
1st) We have to write the equations B and C in order to obtain the final equation A:
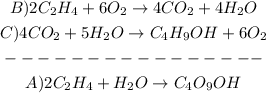
2nd) In this case, the 4 moles of CO2, the 4 moles of H20 and the 6 moles of O2 cancel each other, without the need to multiply or divide the equations. So, we have to add the enthalpy of both equations:
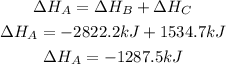
Finally, the enthalpy change for equation A is -1287.5kJ.