Chemistry => Stoichiometry => Limiting reactant
The maximum number of molecules that can be formed will depend on the limiting reactant. The limiting reactant corresponds to the reactant that produces the least amount of product, or in other words, the one that is completely consumed in the reaction.
To find the limiting reactant we are going to divide the moles of each reactant by the stoichiometric coefficients of the balanced equation, the reactant with the lowest ratio will be the limiting reactant.
The balanced equation for this reaction will be:
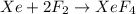
We have, according to the image the following number of molecules:
Xe=2molecules
F2=4molecules
The limiting reactant will be:
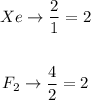
Both reactants are limiting reactants. We can take any reactant and we will have the same number of molecules of XeF4 formed. The ratio Xe to XeF4 is 1/1, so the molecules of XeF4 that can be formed will be:
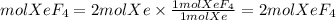
Answer: 2 XeF4 molecules