Step 1
Boyle's law states that the pressure of a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and the amount of the gas remain constant.
Mathematically:
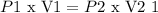
-----------------
Step 2
Information provided:
P1 = pressure 1 = 2.5 atm
V1 = volume 1 = 24.6 L
-------
P2 = unknown
V2 = 18.7 L
----------------
Step 3
P2 is found from (1):
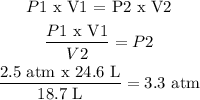
Answer: P2 = 3.3 atm