Answer
394.23 grams
Step-by-step explanation
Given:
1.5 moles BeI₂
What to find:
To convert 1.5 moles BeI₂ to grams.
Solution:
The given moles of BeI₂ can be converted to grams using the mole formula below:
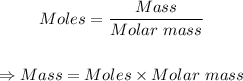
From the periodic table, atomic masses of: (Be = 9.012, I = 126.904)
So molar mass of BeI₂ = 9.012 + (2 x 126.904) = 9.012 + 253.808
Molar mass of BeI₂ = 262.82 g/mol
Now, plug in moles = 1.5 mol, and molar mass = 262.82 g/mol into the formula:
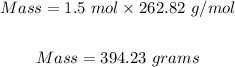
Therefore, 1.5 moles BeI₂ to grams = 394.23 grams