The property that relates number of moles and mass of an atom is the atomic weight. For atoms, we can consult the atomic weight in a periodic table, for example.
Consulting the atomic weight of aluminium, we get:
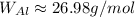
The relationship is as follows:
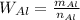
Solving for n and substituting the given mass and the consulted atomic weight, we get:
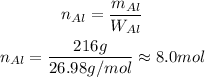
So, there is approximately 8 mol in 216 g of aluminium.