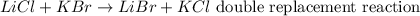Answer:
![\begin{gathered} L\imaginaryI Cl+KBr\operatorname{\rightarrow}L\imaginaryI Br+KCl\operatorname{\lparen}\text{double replacement react}\imaginaryI\text{on}\operatorname{\rparen} \\ C+O_2\operatorname{\rightarrow}CO_2(comb\imaginaryI nat\imaginaryI on\text{ react}\imaginaryI\text{on}) \\ 2H_2O\operatorname{\rightarrow}2H_2+O_2\operatorname{\lparen}\text{decompos}\imaginaryI\text{t}\imaginaryI\text{on react}\imaginaryI\text{on}\operatorname{\rparen} \\ Mg+2AgF\operatorname{\rightarrow}2Ag+MgF_2\operatorname{\lparen}\text{s}\imaginaryI\text{ngle replacement}\operatorname{\rparen} \end{gathered}]()
Explanations:
Combination reaction is the combination of two or more elements to form a compound. From the listed equations, the the reaction that undergoes combination reaction will be:
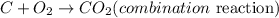
For decomposition reaction, a compound is separated into its constituent elements. An example of a decomposition reaction will be:
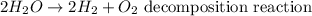
For a single replacement reaction, two compounds are combined to produce an element and a compound. An example of a single replacement reaction will be:
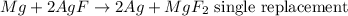
A double replacement reaction involves the reaction between two soluble compounds to produce one soluble and one insoluble products. A double decomposition reaction will be: