Answer:
26.58 atm
Step-by-step explanation:
By the ideal gas law, we have that:
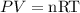
Where P is the pressure, V is the volume, n is the number of moles, R is a constant equal to 0.08206 L atm/ mol K, and T is the temperature.
If we have 32 grams of water vapor, the number of moles will be equal to:
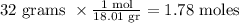
Because 18.01 gr is the molar mass of the vapor water.
On the other hand, the standard temperature is 273 K. So, replacing the values for each constant, we get:
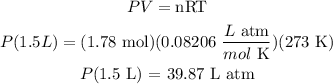
So, dividing both sides by 1.5 L:
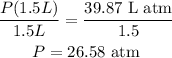
Therefore, the pressure is 26.58 atm.