Answer:
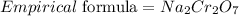
Step-by-step explanation
Given the following parameters
Massof sodium = 29.84g
Mass of Chromium = 67.49grams
Mass of Oxygen = 72.67grams
Determine the moles of the elements
Moles of sodium = 29.84/23 = 1.297moles
Moles of Chromium =67.49/52 = 1.297moles
Moles of Oxygen = 72.67/16 = 4.542moles
Divide through by the lowest number of moles
For Sodium: 1.297/1.297 = 1
For Chromium: 1.2971.297 = 1
For Oxygen: 4.542/1.297 = 3.5
Write all coefficients as whole number
For Sodium: 1(2) = 2
For Chromium: 1(2) = 2
For Oxygen: 2(3.5) = 7
Find the empirical formula
The empirical formula for the compound will be Na2Cr2O7