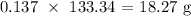Answer:
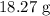
Step-by-step explanation:
Here, we want to get the amount of aluminum chloride produced
We start by writing the equation of the reaction
We have this as:
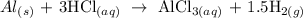
From the equation of reaction, 3 moles of HCl produced 1 mole of AlCl3
The above is the theoretical number of moles
Now, let us get the actual
We start by getting the number of moles of HCl
We can get that by dividing the mass of HCl given by its molar mass
The molar mass of HCl is 36.5 g/mol
Thus, we have the number of moles reacted as:
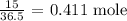
The number of moles of aluminum chloride produced from this is one-third
Mathematically, we have that as:
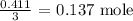
To get the mass of aluminum chloride produced, we have to multiply the number of moles by the molar mass of aluminum chloride
The molar mass of aluminum chloride is 133.34 g/mol
Thus, we have the mass as: