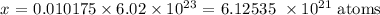Answer:
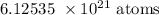
Step-by-step explanation:
Here, we want to get the number of atoms in the given Tungsten mass
Mathematically:
1 mole of Tungsten has a mass of 184g
x moles of Tungsten will have a mass of 1.8722g
Thus:
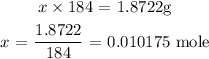
1 mole contains 6.02 * 10^23 atoms
0.010175 mole contains x atoms
Thus: