1) Acidic solution. An acidic solution has a high concentration of H+ ions.
2) Basic solution. A basic solution has a high concentration of OH- ions.
3) HCl chemical reaction.
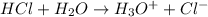
You can also write it as follows
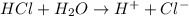
4) pH scale.
When the concentration of H+ is high, the solution is acidic. H+ lowers the pH of the solution.
Option D.