To find the density, we need to know the value of mass and volume. We can find the volume of the cube, using the formula of cube's volume:
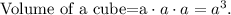
Where a is the value of side, in this case, is 15.6 mm:
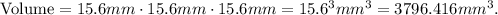
Now, let's convert the volume from mm^3 to cm^3. Remember that 1 cm^3 equals 1000 mm^3:
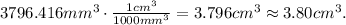
And we can replace the data that we have in the formula of density:
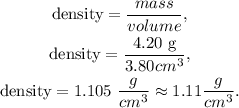
The answer is that the density of the cube of aluminum of side 15.6 mm is 1.11 g/cm^3.