The question requires us to identify the spectator ions for the following chemical reaction:
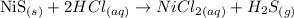
To answer the question, we need to write the net ionic reaction.
The reaction with the ionic species, considering only those molecules in aqueous medium, would be:
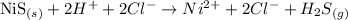
Note that we can "eliminate" the ions Cl- because there is the same amount of it on both sides of the equation:
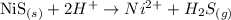
Therefore, since the ions Cl- can be eliminated and are not part of the net ionic equation, they are the spectator ions for this chemical reaction.