Answer:
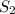
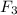 , the empical formula
, the empical formula
Step-by-step explanation:
Let's assume we have 100 grams of the compound:
The 100 grams will consist of:
25.24 grams of S
74.76 grams of F
100.0 grams
We need to find the numbers of atoms in each of these masses. Divide each by that element's molar mass to find atoms of each:
Sulfur: (25.24 g)/(32 g/mole S) = 0.789 moles S
Flourine: (74.76 g)/(19.0 g/mole F) = 5.93 moles F
There are 0.8 atom of S for every 6 atoms of F
We can;t have 0.8 atom of anything, so let's multiply it by a factor to make it a whole number. 5 is the best I can find. Both need to be increased by the same factor:
That makes 4 atoms of S and 30 atoms of fluorine.
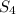
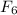
We can reduce that to
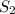
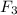 , the empical formula
, the empical formula