3415.2 grams
Step-by-step explanation
to solve this we can use a rule of three
Step 1
Let
water= 100-84%= 16%
so,
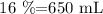
for the water , 1 mL = 1 gram , so
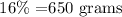
now, let represents the mass of the aluminiu, so
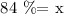
a) the ratio is the same, so we have a proportion
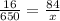
Step 2
finally, solve for x
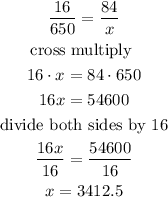
so, the mass of the aluminum is
3415.2 grams