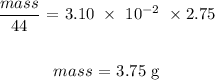Answer:
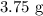
Step-by-step explanation:
Here, we want to calculate the mass that would dissolve
Mathematically:
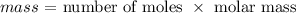
The molar mass of carbon (iv) oxide is 44 g/mol
Thus, we have the number of moles as:
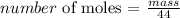
According to Henry's law:
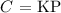
Where;
C is the concentration of dissolved gas
K is the Henry's constant
P is the pressure
Now, from the question, we have:
k = 3.2 * 10^-2
P = 2.75
C is the number of moles per liter which we will represent by the number of moles
Thus, we have it that: