To find the number of moles of a compound given its mass, we can use the Molar Mass of that compound.
In this case, we have 15.0 g of krypton, which is an atom, so we can consult its molar mass from a periodic table.
Consulting it, we get:
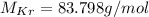
The molar mass, the mass and the atomic number are related by the following:
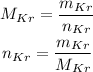
Substituting the values we have:
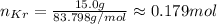
Thus, there is approximately 0.179 mol in 15.0 g of krypton.