Answer
3.0 mol
Step-by-step explanation
Given:
Moles of KI = 6.0 mol
Chemical reaction:
What to find:
The moles of lead iodide (PbI₂) produced.
Step-by-step solution:
From the given balanced chemical reaction above;
2 mol KI produced 1 mol PbI₂,
so 6.0 mol KI will produce:
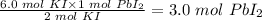
The number of moles of PbI₂ produced = 3.0 mol