So,
We're going to assume a 100g mass of substance, and then interrogate the molar quantities of each element. The steps that we're going to follow, are:
Step 1. Divide the percentage of each element by its molecular mass: (These are the moles)
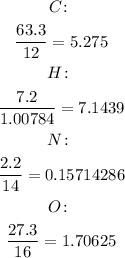
Step 2. We're going to divide each result by the smallest, in this case, it is 0.157.

Now, approximate to the nearest number.
Finally, we obtain:
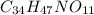
The mass of this compound is 645.315g/mol. So, the empirical and molecular formulas are the same.