In order to find the factor that the pressure will decrease or increase, we need to analyse the following equation:
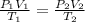
This equation means the relation PV/T is always constant, where P is the pressure, V is the volume and T is the temperature.
So, using the given information, we have:
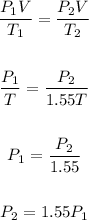
That means the pressure will increase by 1.55x.