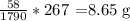C3H60 (I) + 4 02 (g) ----> 3CO2(g) + 3H2O (g)
So, 1 mole of acetone (C3H6O) gives -1790 kj/mol of heat
1 mole of acetone (58g) of acetone gives -1790 kJ/ mol
Now, using unitary method
3.48g of acetone will give
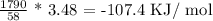
This answers a)
b) Since, 1790 kJ of heat is released by 58 g of acetone
267 kJ of heat will be released by