ANSWER
The percentage composition by mass of magnesium in magnesium sulfate is 20.19%
The percentage composition by mass of sulfur is 26.64%
The percentage composition by mass of oxygen is 53.14%
Explanation:
Firstly, we need to write the chemical formula of magnesium sulfate
The chemical formula of magnesium sulfate is written below as
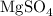
The next step is to calculate the molar mass of magnesium sulfate
According to the periodic table, the molar mass of magnesium sulfate is given as 120.366 g/mol
The next step is to find the percentage composition by mass of each of the elements by using the below formula
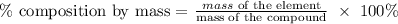
Recall that, the molar mass of magnesium is 24.305g/mol, the molar mass of sulfur is 32.065g/mol, and the molar mass of oxygen is 15.99g/mol
For magnesium
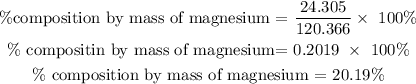
Therefore, the percentage composition by mass of magnesium in magnesium sulfate is 20.19%
For Sulfur
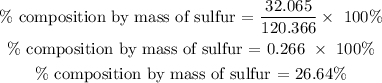
Therefore, the percentage composition by mass of sulfur is 26.64%
for Oxygen
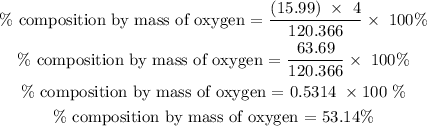
Therefore, the percentage composition by mass of oxygen is 53.14%