We know that the energy of electromagnetic radiation is given by:
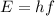
where E is the energy, h is Planck's constant and f is the frequency. Before we can use this formula we need to convert the amount of energy given to J so let's do that:
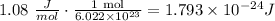
Now that we have the energy of the radiation, we plug it on the energy equation and solve for the frequency:
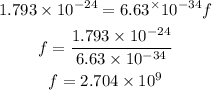
Therefore, the frequency of the cell phone electromagnetic radiation is:
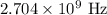
Now that we know the frequency we just need to remember that the frequency and wavelength of electromagnetic radiation are related by:
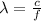
Then we have:
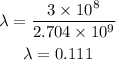
Therefore, the wavelength is 0.111 m