Answer
Specific heat of metal, c = 0.402 J/g°C
Step-by-step explanation
Given:
Mass of the metal = 59.047 g
Initial temperature of the metal = 100.0°C
Initial temperature of water = 23.7°C
Final temperature of water = 27.8°C
Final temperature of the metal = 27.8°C
Volume of water = 100.0 mL
Required: Specific heat of the metal
We know: specific heat of water = 4.184 J/g°C
Density of water = 1 g/mL
Solution
Step 1: Find the mass of water using density and volume given
Mass = density x volume
Mass = 1 g/mL x 100 mL
Mass = 100 g
Step 2: Calculate the specific heat of the metal
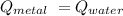
Q = m x c x Δ T
Therefore:
[m x c x Δ T] of metal = [m x c x Δ T] of water
59.047 g x c x (100-27.8 °C) = 100 g x 4.184 J/g°C x (27.8-23.7 °C)
c = 0.402 J/g°C