Answer:
480K
Explanations:
According to the Gay Lussac's law, the pressure of a given mass of gas is directly proportional to the temperature provided that the volume remains constant. Mathematically;
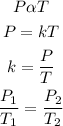
where:
P₁ and P₂ are the initial and final pressure
T₁ and T₂ are the initial and final temperature (in kelvin)
Given the following parameters;
P₁ = 500 torrs
P₂ = 800 torrs
T₁ = 27 + 273 = 300K
Required
Final temperature T₂
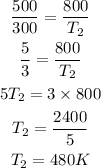
Therefore the temperature that changes the pressure to 800 torrs is 480K